 |
Preparation |
| Home |
| The most common method to prepare polyurethanes is by
reacting a polyol with a di or polyisocyanate. |
R-OH + R1-NCO ® R-OCONH-R1 |
2 R1-NCO + H2O
® R1-NH-COOH
® R1-NHCONH-R1
+ CO2 |
R1-NCO + R-NH2 ®
R1-NHCONH-R |
3 R-NCO ® |
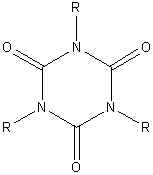
|
Isocyanurate ring |
|
| The polyurethanes can be light
stable if the isocyanates used are aliphatic or cycloaliphatic in nature. In addition the
polyol has to be light stable. For lower cost applications or where light stability is not
important aromatic isocyanates can be used. Aromatic isocyanates are faster reacting under
most conditions than aliphatic isocyanates. The urethane linkage is very polar and strongly
hydrogen bonded. This hydrogen bonding leads to an increase in the glass transition
temperature (Tg) of the polymer and to a change in solubility of the polymer. The high
cohesive energy if the urethane groups is 6294 cal/mol or 26370 J/mol. The different
solubility parameter of the urethane linkage leads also to a phase separation and to the
formation of polar hydrogen bonded domains. Polymers high in urethane groups can be
dissolved in solvents also strong in hydrogen bonding capability such as
1-methyl-2-pyrrolidone (CAS # 872-50-4). Crosslinked polymers and foams can be swollen in
this solvent. The reaction of a polyol with an isocyanate requires in most applications
a catalyst. Organotin and
non-tin compounds can
be used for the catalysis of aliphatic and aromatic isocyanates. Aromatic
isocyanates are catalyzed
with t-amines. |
| Urea linkages can be formed by the
reaction of water with isocyanates, as a side reaction also carbon dioxide is
produced. This reaction can be a problem if void a free polyurethanes is to
be prepared. This
reaction can be used to advantage in the preparation of foams. In the
preparation of foams blown with carbon dioxide special
t-amine catalysts are used to achieve the high reaction rate of the
aromatic isocyanate with water. The urea linkages formed
from isocyanates and water is a extremely polar and a strongly hydrogen bonded group. This
group is even more prone to phase separation and to be incompatible with the remaining
polymer. The cohesive energy of the urea group is 10,000 cal/mol or 41,900 J/mol.
Solubility of urea group containing polyurethanes is even poorer than only urethane
containing polymers. The high glass transition temperature of polymers prepared with urea
groups leads to a high heat distortion temperature of polymers. The linkage is also
very stable to hydrolysis under basic and acidic conditions. If the urea groups is formed
from aliphatic isocyanates the polymers are also very light stable. A high content of urea
groups can lead in coatings or moldings to lower gloss. If the formation of urea is a side
-reaction and has not been considered in the molar ratio of polyol to isocyanate it can
lead in low functional polymers to poor resistance properties. Direct
formation of polyureas is also possible by reacting a polyamine with an
isocyanate. The reaction is extremely fast and with primary sterically non-hindered amine
almost explosive. Using sterically hindered amines or amines with a lower pKa value the
reaction speed is more manageable. Because of the high reaction speed the application of
such as system is in plural component spray applications where the components are
mixed in the spray zone. Also RIM (reaction injection molding) compounds can be prepared
by this reaction. Polymers prepared by this reaction have a high heat distortion
temperature. |
| The trimerization of isocyanates in the
presence of certain catalysts
leads to an isocyanurate ring which is a very temperature stable link. This reaction can
be used to create temperature stable polymers with high flame resistance. By combining
conventional polyurethane reaction with isocyanurates flame resistant foams can be
prepared. The heat distortion temperature of polyurethane RIM formulations can also be
improved with isocyanurate crosslinks. |
| The reaction rate of a polyol with an isocyanate is very slow in the
absence of a catalyst. Many compounds can catalyze the reaction of an isocyanate with a
polyol. Typically in polyurethane systems, dialkyltin,
lead octoate, zinc octoate and
amines are used. For aromatic isocyanates amines are very effective catalysts. Dibutyltin
dilaurate (DBTDL) is a standard catalyst for aliphatic and cycloaliphatic isocyanates.
Catalysts which catalyze the hydroxyl reaction also catalyze the water reaction. DBTDL
shows some preference for the hydroxyl over the water reaction. Amines on the other hand
preferentially catalyze the water over the hydroxyl reaction. |
|
|
|
|
Last edited on:
|
November 14, 2006
|
Copyright®, Design, Layout and Technical Content by:
|

|
|
|
