|
No chemistry required (it would not hurt to know some)
|
|
Some chemistry or physics required
|
|
| Catalysts |
|
|
|
|
|
|
|
Amine Catalysts
|
|
|
|
The amine catalysts used in the preparation of polyurethanes
are t-amines with multiple functionality. These amine catalyst are excellent
for catalyzing the water reaction in aromatic isocyanate foams. The are
commonly called "blowing" catalysts because they accelerate
the water reaction and the formation of carbon dioxide.
|
|
|
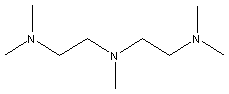
PMDETA is an excellent catalyst for the preparation of
partially blown rigid foams or moldings. There is large variety of
multi-amine functional catalysts available.
|
|
|
Evaporation of amines from foams is one of the factors for
fogging of car windows and for "amine odor" of foams. Hydroxyl
group containing catalysts can reduce volatility and odor.
|
|
|
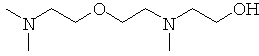
This an example of a t-amine catalyst with
hydroxyl functionality.
|
|
|
Catalysts which favor the reaction of an isocyanate with a
hydroxyl group are called gelling catalysts. For the formation of a good
foam a balance between gelation and blowing is required.
|
|
|
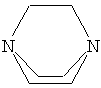
Diethylenetriamine is one of the few amines which give good
gelation properties.
|
|
|
Quaternary ammonium compounds can function as trimerization
catalysts
|
|
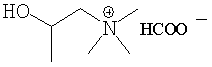
|
The formate and the 2-ethylexanoate are delayed action
trimerization catalysts.
|
|
|
Metal Catalyst
|
|
|
|
|
Organotin compounds, specially dibutyltin dilaurate and stannous
octoate are the most often used metal catalysts. Dibutyltin dilaurate is a
strong gelation catalyst, selective for hydroxyl reaction over
water reaction. Stannous octoate is the standard tin catalyst
for slabstock application.
|
|
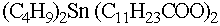
|
There is a concern about organotin catalysts in the
environment.
|
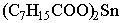
|
Not considered an organotin compound, low in toxicity. This
compound is actually the 2-ethylhexanoate.
|
|
|
Some of the potassium salts are excellent trimerization
catalysts and are used in the preparation of isocyanurate foams used in
flame retardant applications.
|
|
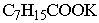
|
Besides the octoate also the acetate salt acts as a
trimerization catalyst
|
|
|
|
|
|
|
|
|
|
|
Last edited on:
|
November 20, 2006
|
Copyright®, Design, Layout and Technical Content by:
|

|
|
|