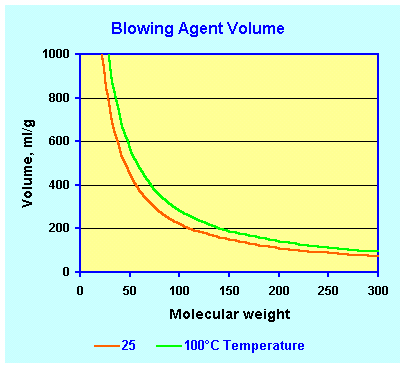
| This graph shows the volume of gas
generated (ml) by 1 gram of blowing agent. Look up the molecular
weight (MW) of the blowing agent in the table and
look up in the graph below the amount of gas the molecular weight
corresponds to. For example if you use HFC 134, you will find in the
table a MW of 102. You will find that one gram of HFC 134 creates
approximately slightly more than 200 ml of gas at room temperature and
atmospheric pressure. At 100°C this
would be approximately 280 ml. If you use water as a blowing agent,
the MW is 18, but water is not the blowing agent, it is carbon
dioxide. Therefore the MW of carbon dioxide is 44 as shown in the
table. I rather have the data in
ft3/lb. |
 |
| The volume of gas generated from the evaporation of a compound
is related to the molecular weight of the compound. One gram mol of gas occupies
approximately 22.4 liter of volume at room temperature at and atmospheric pressure. One
pound mol of a gas occupies approximately 360 cubic feet of volume at room
temperature
and at atmospheric pressure. Therefore the amount of gas generated by the evaporation of a
compound is inversely related to the molecular weight. A lower molecular weight blowing
agent is a more efficient product. The actual efficiency of the blowing agent is in many
applications about 70 % of theoretical. |
|
| To use this table look up the molecular weight and determine in the above
graph how much gas volume is generated with a given blowing agent. One gram of a
blowing agent with a molecular weight of 50 will for example form 400 ml of gas at
atmospheric pressure at room temperature and about 500 ml at 100ºC. |
A one weight
percent addition of blowing agent with a molecular weight of 50 will increase the volume
of 100 gram of a foam to 400 ml of gas plus the volume polyurethane (100/specific gravity)
at room temperature. A theoretical increase in volume of about 5 fold. The actual
increase is going to be smaller in the range of 4.2 to 4.8 fold.
|
| Characteristic of Blowing Agents |
| Molecular
weight |
Name |
Chemical Name |
29 |
|
Air |
34 |
HFC
41 |
Fluoromethane |
40 |
|
Argon |
44 |
|
Carbon
dioxide/Water |
48 |
HFC
161 |
Fluoroethane |
52 |
HFC
32 |
Difluoromethane |
58 |
|
Acetone |
58 |
|
Isobutane |
64 |
HFC
1132 |
1,2-Difluoroethylene |
64 |
HFC
1132a |
1,1-Difluoroethylene |
66 |
HFC
152 |
1,2-Difluoroethane |
66 |
HFC
152a |
1,1-Difluoroethane |
70 |
HFC
23 |
Trifluoromethane
|
70 |
|
Cyclopentane |
72 |
|
Pentane |
72 |
|
Isopentane |
80 |
HCFC
1131a |
1-Chloro-1-fluoroethylene
|
82 |
HFC
1123 |
Trifluoroethylene |
83 |
HCFC
151a |
1-Chloro-1-fluoroethane |
84 |
|
Krypton |
84 |
HCFC
143a |
1,1,1-Trifluoroethane |
84 |
HFC
143 |
1,1,2-Trifluoroethane |
85 |
|
Methane,
dichloro- |
86 |
HCFC
22 |
Chlorodifluoromethane |
98 |
HFC
263fb |
1,1,1-Trifluoropropane |
102 |
HFC
134 |
1,1,2,2-Tetrafluoroethane
|
102 |
HCFC
134a |
1,1,1,2-Tetrafluoroethane |
104 |
R
13 |
Chlorotrifluoromethane |
113 |
HCFC
142b |
1-Chloro-1,1-difluoroethane |
117 |
HCFC
141b |
Ethane,
1,1-dichloro-1-fluoro- |
117 |
HCFC
141 |
1,2-Dichloro-1-fluoroethane |
118 |
HCFC
133 |
1,1,2-Trifluoro-2-chloroethane |
120 |
HCFC
125 |
Ethane,
pentafluoro- |
121 |
R
12 |
Dichlorodifluoromethane |
132 |
HFC
1225zc |
1,1,3,3,3-pentafluoropropene
|
133 |
|
Trichloroethane |
134 |
HCFC-245fa |
1,1,1,3,3,-pentafluoropropane |
134 |
HFC-245ca |
1,1,2,2,3,-pentafluoropropane |
134 |
HFC-245eb |
1,1,1,2,3,-pentafluoropropane |
136 |
HCFC
124a |
Ethane,
1-chloro-1,1,2,2-tetrafluoro- |
137 |
CFC-11 |
Trichlorofluoromethane |
148 |
HFC-365mfc |
1,1,1,3,3-Pentafluorobutane |
150 |
HFC-E
245 |
Difluoromethyl
2,2,2-trifluoroethyl ether |
150 |
HFC
245caEab |
1-(Difluoromethoxy)-1,2,2-trifluoroethane
|
151 |
HCFC
131 |
1,1,2-Trichloro-2-fluoroethane |
151 |
HCFC
131a |
Ethane,
1,1,2-trichloro-1-fluoro- |
152 |
HFC
236ea |
1,1,1,2,3,3-Hexafluoropropane |
152 |
HCFC
236fa |
1,1,1,3,3,3-Hexafluoropropane |
153 |
HCFC
123 |
2,2-Dichloro-1,1,1-trifluoroethane |
153 |
HCFC
123b |
Ethane,
1,1-dichloro-1,2,2-trifluoro- |
168 |
HCFC
235fa |
1,1,1,3,3-Pentafluoro-3-chloropropane |
170 |
HFC
227 |
1,1,1,2,3,3,3-Heptafluoropropane |
185 |
HCFC
234fb |
Propane,
1,1-dichloro-1,3,3,3-tetrafluoro- |
186 |
HCFC
226da |
2-Chloro-1,1,1,3,3,3-hexafluoropropane |
188 |
HFC-C
318 |
Octafluorocyclobutane
|
188 |
HFC
218 |
Octafluoropropane |
203 |
HCFC
225da |
1,1,1,3,3-Pentafluoro-2,3-dichloropropane |
203 |
HCFC
225cb |
1,1,2,2,3-Pentafluoro-1,3-dichloropropane |
221 |
HCFC
216ca |
1,3-Dichloro-1,1,2,2,3,3-hexafluoropropane |
237 |
HCFC
215ca |
1,1,3-Trichloro-1,2,2,3,3-pentafluoropropane
|
282 |
HFC-C
52-11 |
Undecafluorocyclohexane |
|
|
|
|
|
|
Last edited on:
|
November 16, 2006
|
Copyright®, Design, Layout and Technical Content by:
|

|
|
|
|